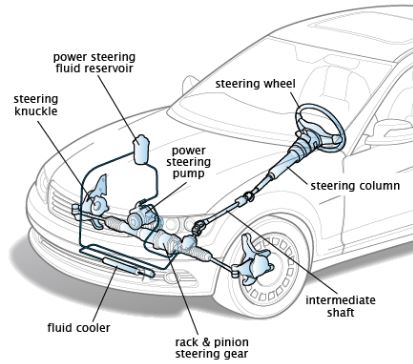
Calorific value:
- Calorific value is defined as the fuel of unit mass or volume is completely burnt at this process the amount of heat liberated is known as calorific value.
Higher Calorific Value:
- It is defined as When the burnt of unit mass is done completely and the product of combustion should be cool down at room temperature.
- This value is shown in cal/g, Kcal/g etc..
Lower Calorific Value:
- It is defined by the process of Unit mass or volume is burnt completely and that the product are permitted to escape is known as Lower Calorific Value.
Equilibrium constant:
- Equilibrium constant is given by the ratio of Rate of forward reaction to the Rate of backward reaction.
- Kc=Forward reaction/Backward reaction.
Please Subscribe! and Don’t forget to Follow us on Facebook, Twitter, Linkedin, Instagram and Google Plus.