Solid Solutions:
It is Known as Solid Solution when two or more type of homogenous atoms exit in the solid state.
There are two types of Solid Solutions.
1. Interstitial solid solution.
2. Substitutional solid solution.
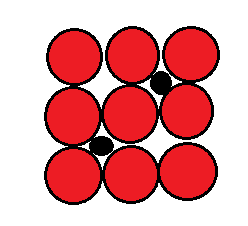
1. Interstitial Solid Solution:
This type of solid solutions which has less than 1-angstrom number are used to form the interstitial solid solution.
And this is formed when the space of lattice structure of large solvent in which the small atomic radii fit into this solvent then the interstitial solid solution are formed.
This solid solution has limited solubility. solid solution can be separated easily and this solid solution has a high melting temperature.
Eg: In this type the carbon dissolving in iron.
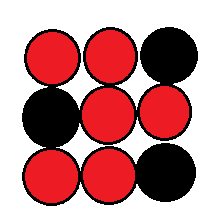
2. Substitutional Solid Solution:
In this type of solid solution, Solute atoms are substituted for solvent atoms in the crystal lattice. in this, the crystal structure does not change but it has some distortion by adding atoms. and forms a solute and solvent diameter difference.
There is no change in lattice structure in the gold-silver alloy.
Please Subscribe! and Don’t forget to Follow us on Facebook, Twitter, Linkedin, Instagram and Google Plus.