Fuel Cell:
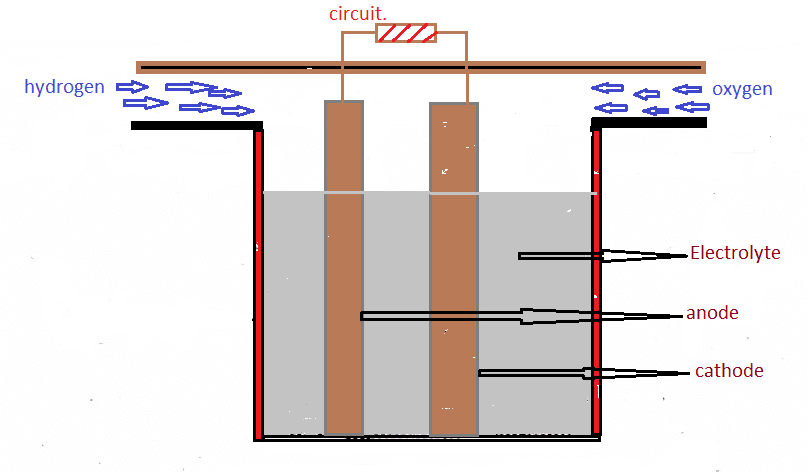
Fuel Cell: Fuel Cell is an Electro-Chemical device. Fuel Cell works same as the battery, which produces electrical energy by the help of the chemical reaction. In the fuel cell, it consists of electrode and electrolyte which is not in battery. the chemical reaction occurs when the fuel supplied to it gets oxidized. In this type of cells, there is the continuous generation of electricity by supplying the fuel to it. In this hydrogen is most efficient and economic one and fuel we can get is highly available in the atmosphere and the emission of this is water. Its efficiency is about 70% and a non-polluting one. This is a good method of generating power for long period.
Storage of hydrogen is difficult due to it should be in liquid or at high pressure, this is because it has low energy density. The maintenance is easy.
Working of Fuel Cell:
When hydrogen is supplied to the anode, it gets oxidized and makes the electrons free, by this they used to flow in the circuit, To get combine with oxygen-hydrogen is used to pass through the electrolyte to the cathode, by this combine reaction it forms into the water.
Advantages of fuel cell:
- High efficiency about 70%.
- Required space is less.
- It does not require Cooling water.
- No pollution.
- Efficiency remained is constant at part load.
- Maintenance cost is less.
- Disadvantages:
- Service life is less.
- Capital cost is high for this.
Please Subscribe! and Don’t forget to Follow us on Facebook, Twitter, Linkedin, Instagram and Google Plus.