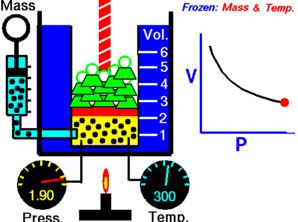
Define the first law of thermodynamics and explain about why it is the law of conservation of energy?
First Law of Thermodynamics In this, it states that both heat and work are mutually convertible. this is also said to be that when the heat is supplied to a system should equal to network done by it. When there is both heat and work is transferred then the net energy stored in the system …