What is Kelving-Planck Statement
The Kelvin-Planck statement is one of the formulations of the second law of thermodynamics and is named after Lord Kelvin and Max Planck, who independently contributed to its development. The statement is specifically associated with the limitations of heat engines.
The Kelvin-Planck statement can be expressed as follows:
No heat engine operating in a cycle can absorb heat from a single reservoir and convert it entirely into work without any other effect.
In simpler terms, it emphasizes that it is impossible to create a heat engine that takes heat from a single source (reservoir) and converts all of it into work without any waste or without affecting other parts of the system.
This statement is closely related to the concept of efficiency in heat engines. The efficiency (η) of a heat engine is defined as the ratio of the useful work output (Wout) to the heat absorbed (Qin):
η=Wout/Qin
The Kelvin-Planck statement implies that there is a limit to the efficiency of a heat engine, and no heat engine can have an efficiency of 100% (i.e., converting all input heat into work) when operating in a cycle.
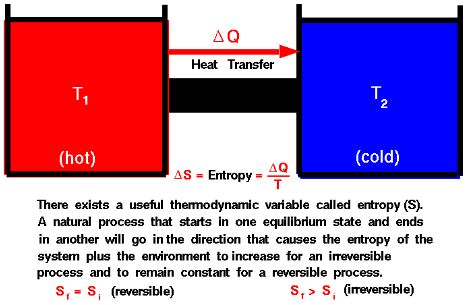
In practical terms, this statement is consistent with our everyday experience and observations of natural processes. It underscores the irreversibility of certain thermodynamic processes and the fact that not all heat energy can be converted into useful work without some portion of it being rejected to a lower-temperature reservoir.
Understanding Kelvin Planck Statement
The Kelvin-Planck statement is a key principle in the field of thermodynamics, specifically expressing a limitation on the efficiency of heat engines. To understand the statement, let’s break down its components and implications:
Heat Engines:
A heat engine is a device that converts heat energy into mechanical work. It operates on the principles of the second law of thermodynamics, which deals with the direction of natural processes.
Heat Reservoirs:
In the context of the Kelvin-Planck statement, we consider heat engines interacting with heat reservoirs. A heat reservoir is an infinite source or sink of heat at a constant temperature.
Efficiency of a Heat Engine:
The efficiency (η) of a heat engine is a measure of how effectively it converts heat into work. It is defined as the ratio of the useful work output to the heat absorbed: η=Wout/Qin.
Kelvin-Planck Statement:
The statement asserts that it is impossible for a heat engine operating in a cycle to absorb heat from a single reservoir and convert all of it into work without any other effects.
Implications:
The statement highlights the idea of irreversibility in natural processes. It emphasizes that not all the heat absorbed by a heat engine can be converted into useful work. Some amount of heat must be rejected to a lower-temperature reservoir.
Practical Example:
Imagine a heat engine taking in heat from a hot reservoir, performing work, and then rejecting some heat to a colder reservoir. The Kelvin-Planck statement asserts that there will always be some heat that cannot be converted into work and must be transferred to a lower-temperature environment.
Clausius Statement Vs.Kelvin-Planck Statement:
The Kelvin-Planck statement is complemented by the Clausius statement of the second law, which focuses on the cyclic operation of a refrigerator and states that it is impossible for any device to operate in a cycle while transferring heat from a cold reservoir to a hot reservoir without external work input.
In summary, the Kelvin-Planck statement emphasizes the inherent limitations on the efficiency of heat engines, pointing out that complete conversion of heat into work without any waste is unattainable in a cyclic process. It underscores the irreversible nature of certain thermodynamic processes and has significant implications for the design and understanding of heat engines.
Practical Applications Kelvin Planck Statement
The Kelvin-Planck statement of the second law of thermodynamics, which states that no heat engine can operate in a cycle and convert all the heat it receives from a single reservoir into work, has several practical applications and implications in various engineering and scientific fields. Here are some practical applications:
- Design and Optimization of Heat Engines:
- The Kelvin-Planck statement guides engineers in the design and optimization of heat engines, helping them set realistic efficiency expectations. It serves as a fundamental principle in thermodynamic analysis for designing efficient power plants, combustion engines, and other heat-based systems.
- Efficiency Analysis in Power Plants:
- Power plants, which convert heat from burning fossil fuels or other sources into electricity, adhere to the principles of the Kelvin-Planck statement. Understanding the limitations imposed by this statement is crucial for evaluating and improving the efficiency of power generation.
- Automotive Engines:
- The statement is relevant in the design and improvement of internal combustion engines used in automobiles. Engineers must consider the limitations of heat-to-work conversion when optimizing fuel efficiency and performance.
- Refrigeration and Air Conditioning Systems:
- In the field of refrigeration and air conditioning, the Kelvin-Planck statement is applied to optimize systems that transfer heat from a lower-temperature space (e.g., inside a refrigerator) to a higher-temperature space (e.g., the surrounding room) while requiring external work input.
- Energy Recovery Systems:
- Systems that recover waste heat for additional useful work, such as combined heat and power (CHP) systems, must take into account the principles of the Kelvin-Planck statement. These systems aim to maximize the utilization of heat energy that would otherwise be lost.
- Thermoelectric Devices:
- The development of thermoelectric devices, which convert heat differentials into electrical energy, involves considerations based on the Kelvin-Planck statement. These devices are used in various applications, including waste heat recovery.
- Geothermal Power Plants:
- Geothermal power plants, which harness heat from the Earth’s interior, must comply with the principles outlined in the Kelvin-Planck statement when converting geothermal heat into electricity.
- Energy Policy and Sustainability:
- Understanding the limitations imposed by the Kelvin-Planck statement is crucial for policymakers and energy planners when formulating sustainable energy strategies. It influences decisions on energy sources, efficiency measures, and environmental impact assessments.
- Research and Development:
- Researchers in thermodynamics and energy engineering use the Kelvin-Planck statement as a foundational principle for developing new technologies and improving existing systems, aiming to maximize energy efficiency.
- Educational and Training Tools:
- The Kelvin-Planck statement is a key concept taught in thermodynamics courses, providing students and professionals with a fundamental understanding of the constraints on heat-to-work conversion in practical applications.
In summary, the Kelvin-Planck statement plays a crucial role in guiding the design, analysis, and optimization of various systems and technologies that involve the conversion of heat into work. It helps set realistic expectations for efficiency and drives advancements in energy-related fields.
Frequently Asked Questions
1.What is the Kelvin-Planck statement in thermodynamics?
The Kelvin-Planck statement asserts that no heat engine operating in a cycle can absorb heat from a single reservoir and convert it entirely into work without any other effect.
2.How does the Kelvin-Planck statement relate to the efficiency of heat engines?
The statement implies that there is a limit to the efficiency of heat engines, suggesting that it is impossible for a heat engine to convert all absorbed heat into work in a cyclic process.
3.Why is it impossible for a heat engine to achieve 100% efficiency according to the Kelvin-Planck statement?
The statement highlights the inherent irreversibility of certain thermodynamic processes, indicating that not all heat can be converted into work without some portion being rejected to a lower-temperature reservoir.
4.Are there practical examples illustrating the Kelvin-Planck statement?
Yes, practical examples include heat engines, such as car engines and power plants, where not all absorbed heat can be converted into mechanical work due to the limitations outlined in the Kelvin-Planck statement.
5.How does the Kelvin-Planck statement impact the design of power plants?
Engineers designing power plants use the Kelvin-Planck statement to set realistic expectations for the efficiency of the plant, considering the fact that not all heat can be converted into electricity.
6.Does the Kelvin-Planck statement apply to refrigeration systems?
Yes, the Kelvin-Planck statement is applicable to refrigeration systems, where heat is transferred from a lower-temperature space to a higher-temperature space with the input of external work.
7.Can the Kelvin-Planck statement be violated in any practical scenario?
No, the Kelvin-Planck statement is a fundamental principle in thermodynamics, and there is no known practical scenario where it can be violated.
8.How is the Kelvin-Planck statement different from the Clausius statement?
The Kelvin-Planck statement focuses on the limitations of heat engines, while the Clausius statement deals with the limitations of refrigeration systems, stating that no refrigerator can operate in a cycle without external work input.
9.What role does the Kelvin-Planck statement play in the study of thermodynamics?
The statement is a fundamental concept in thermodynamics, guiding the understanding of energy transformations in heat engines and influencing the analysis of energy conversion processes.
10.Can improvements in technology overcome the limitations posed by the Kelvin-Planck statement?
A: While technological advancements can enhance the efficiency of heat engines, the Kelvin-Planck statement sets a theoretical limit, indicating that complete conversion of heat into work without any waste is unattainable in a cyclic process.