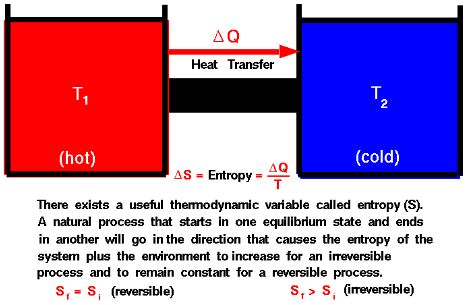
Introduction
Thermodynamics is the branch of physics that deals with the study of energy and its transformations within a system. It provides a framework for understanding the behavior of matter and energy, particularly in relation to heat and work. The laws of thermodynamics govern the fundamental principles that dictate the direction and efficiency of energy exchanges. The first law, known as the law of conservation of energy, states that energy cannot be created or destroyed, only transferred or converted between different forms. The second law introduces the concept of entropy, describing the tendency of systems to move towards increased disorder. Thermodynamics plays a crucial role in various scientific and engineering disciplines, influencing the design and optimization of energy systems, engines, and processes across a wide range of applications.
What is Thermodynamic System?
A thermodynamic system is a well-defined region in space containing a certain amount of matter or substance that is the focus of a thermodynamic analysis. The system can be as simple as a gas inside a container or as complex as an entire steam power plant. The boundaries of the system separate it from its surroundings, which include everything external to the system.
The description of a thermodynamic system often involves specifying its properties, such as temperature, pressure, volume, and composition. These properties can undergo changes, and the study of these changes is crucial in understanding the behavior of the system. Thermodynamic systems are commonly classified into three main types based on the nature of energy and matter exchange with their surroundings
Classification of Thermodynamic Systems
Thermodynamic systems can be broadly classified into three main types:
closed systems, open systems, and isolated systems. Each type has distinct characteristics in terms of the exchange of energy and matter with the surroundings.
- Closed System:
- Definition: A closed system is a thermodynamic system that allows the exchange of energy with its surroundings, typically in the form of heat or work, but does not permit the transfer of matter across its boundaries.
- Characteristics:
- The total mass of the system remains constant.
- Energy transfer in the form of heat or work is possible across the system boundaries.
- Common examples include a piston-cylinder arrangement containing a gas, where the gas can undergo changes in pressure and temperature without any mass entering or leaving the system.
- Open System:
- Definition: An open system is a thermodynamic system that allows both the exchange of energy and matter with its surroundings.
- Characteristics:
- Energy can be transferred in the form of heat or work.
- The system can exchange mass with its surroundings, meaning substances can enter or exit the system.
- Examples of open systems include a steam power plant, where water and steam can flow in and out of the system, and the system interacts with its surroundings by exchanging heat and performing work.
- Isolated System:
- Definition: An isolated system is a thermodynamic system that does not exchange energy or matter with its surroundings.
- Characteristics:
- The total energy and mass within the system remain constant over time.
- Isolated systems are theoretical constructs used for certain analyses, as it is challenging to find a perfectly isolated system in practice.
- A well-insulated, sealed container can be considered an isolated system for practical purposes, as it minimizes energy exchange with the surroundings.
Classification of Thermodynamic Properties
These classifications provide a basis for understanding and analyzing the behavior of thermodynamic systems, allowing scientists and engineers to model and optimize processes in various applications, from heat engines and refrigeration systems to chemical reactions and power plants.
Thermodynamic system properties are characteristics or quantities that describe the state and behavior of a thermodynamic system. These properties help in analyzing and understanding the thermodynamic processes that occur within the system. The properties can be broadly categorized into two main types: intensive and extensive.
- Intensive Properties:
- Definition: Intensive properties are independent of the size or mass of the system. They are inherent to the substance and remain constant throughout the entire system, regardless of its size.
- Examples: Temperature, pressure, density, specific volume, and chemical composition are common intensive properties. For instance, the temperature of a gas in a container is the same throughout the container, irrespective of the size or quantity of the gas.
- Extensive Properties:
- Definition: Extensive properties depend on the size or mass of the system. As the size of the system increases, so does the value of the extensive property.
- Examples: Mass, volume, energy, and entropy are extensive properties. For example, the total energy of a system is the sum of the energies associated with its individual components. Similarly, the volume of a gas is directly proportional to the quantity of the gas.
In addition to this classification, properties can also be categorized as either state or path functions:
- State Functions:
- Definition: State functions depend only on the current state of the system and not on the path by which the system reached that state. They are determined by the system’s condition and are independent of the process history.
- Examples: Internal energy, enthalpy, temperature, pressure, and density are state functions. These properties are crucial for defining the equilibrium state of a system.
- Path Functions:
- Definition: Path functions are properties that depend on the path taken during a process. They are influenced by the specific route the system follows from one state to another.
- Examples: Work and heat are path functions. The work done on or by a system and the heat transferred during a process depend on the details of how the process occurs.
Understanding and utilizing these thermodynamic system properties are fundamental to the analysis and design of various engineering systems, including heat engines, refrigeration systems, and chemical processes. They provide a foundation for applying the laws of thermodynamics and modeling the behavior of complex systems in a variety of practical applications.
Advantages of Applying Thermodynamics System
- Engineering and Design: Thermodynamics provides a fundamental framework for engineers and scientists to design and optimize various systems, such as engines, refrigeration systems, and chemical processes.
- Energy Efficiency: Understanding thermodynamics allows for the development of energy-efficient technologies and processes, leading to improved performance and reduced energy consumption.
- Predictive Capability: Thermodynamics enables the prediction of the behavior of materials and substances under different conditions, helping in the design and analysis of systems before they are actually built or implemented.
- Environmental Impact: Thermodynamic principles contribute to the development of environmentally sustainable practices by optimizing energy usage and minimizing waste in industrial processes.
Disadvantages or Challenges of Thermodynamics System
- Complexity: Thermodynamics can be a complex field, especially when dealing with real-world systems. Theoretical concepts might need to be simplified for practical applications, leading to some loss of accuracy.
- Assumptions and Idealizations: Many thermodynamic analyses rely on assumptions and idealizations that may not perfectly represent the complexities of actual systems. These simplifications can sometimes lead to deviations from real-world behavior.
- Limited Applicability: Thermodynamics has certain limitations, especially in cases involving very small scales (nanoscale) or extremely high speeds (relativistic speeds), where other branches of physics, such as quantum mechanics or relativistic physics, become more relevant.
- Initial Conditions: The analysis of thermodynamic systems often assumes certain initial conditions, and deviations from these conditions can introduce uncertainties in predictions. Real-world conditions may vary from idealized scenarios.
In summary, while thermodynamics provides a powerful tool for understanding and optimizing various systems, its application is not without challenges. The advantages lie in its predictive capability and contributions to efficient engineering practices, while the disadvantages include complexities, assumptions, and limitations in certain scenarios.
Frequently Asked Questions – FAQ’s
Can thermodynamics be applied to everyday scenarios?
Yes, thermodynamics concepts are present in various everyday situations, from understanding household appliances to the functioning of the human body, showcasing the widespread applicability of thermodynamic principles.
Are there limitations to thermodynamics?
Yes, thermodynamics has limitations, particularly in extreme conditions such as the nano-scale or relativistic speeds, where other branches of physics become more relevant.
How does thermodynamics contribute to environmental sustainability?
Thermodynamics aids in the development of environmentally sustainable practices by optimizing energy usage and minimizing waste in industrial processes.
Why is thermodynamics important in engineering?
Thermodynamics is crucial in engineering for designing and optimizing systems, ensuring energy efficiency, and predicting the behavior of materials and substances.
What is the significance of an isolated system in thermodynamics?
An isolated system, though theoretical, helps in understanding systems where neither energy nor matter is exchanged with the surroundings, contributing to foundational thermodynamic concepts.
How do state functions differ from path functions?
State functions, such as internal energy and enthalpy, depend only on the current state of the system, while path functions, like work and heat, depend on the specific path taken during a process.
Can you provide examples of extensive properties?
Extensive properties, like mass and volume, depend on the size or quantity of the system, increasing proportionally as the system’s size increases.
What is a thermodynamic system?
A thermodynamic system is a well-defined region in space that is the focus of thermodynamic analysis, encompassing a certain quantity of matter and energy.
How are thermodynamic systems classified?
Thermodynamic systems are classified into closed, open, and isolated systems based on their interactions with the surroundings in terms of energy and matter exchange.
What are intensive properties of a thermodynamic system?
Intensive properties, such as temperature and pressure, are characteristics that remain constant throughout the entire system, independent of its size or quantity.